I-COVID 19
-
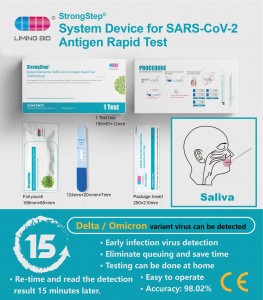
Idivaysi yesistimu ye-GrostStep ye-SARS-Cov-2 Antigen Rapid Test
Inkonti encane 500210 Ukucacisa 1 isivivinyo / ibhokisi Isimiso sokutholwa Immunochrochrographic assay Izindola AmatheUkusetshenziswa okuhlosiwe I-strongstepIdivaysi yesistimu ye-SARS-Cov-2Antigen Rapid Test isebenzisa ubuchwepheshe be-Immunochromatography ukuthola i-SARS-Cov-2 Nucleocapsopid Antigen ngamathe abantu. Lokhu kuhlola kusetshenziswa okukodwa kuphela futhi kuhloswe ukuzihlola. Kunconywa ukusebenzisa lolu vivinyo kungakapheli izinsuku eziyi-7 zokuqala uphawu. I-LT isekelwa ukuhlolwa kokusebenza kokudla. -

I-SARS-Cov-2 Antigen Rapid Test (Nasal)
Inkonti encane 500200 Ukucacisa 1 izivivinyo / ibhokisi; izivivinyo / ibhokisi eli-5; izivivinyo / ibhokisi eli-20 Isimiso sokutholwa Immunochrochrographic assay Izindola I-Anterior Nasal Swab Ukusetshenziswa okuhlosiwe I-ConfSTep® SARS-Cov-2 Antigen Suptope Cessette isebenzisa ubuchwepheshe be-Immunochromatography ukuthola i-SARS- I-Antigen ye-NUCLEOCACAppID e-Anterior Nasal Swab specimen yabantu. Le testis ukusetshenziswa okukodwa kuphela futhi ihloselwe ukuzihlola. Kunconywa ukusebenzisa lolu vivinyo kungakapheli izinsuku ezi-5 zokuqala uphawu. Isekelwa ukuhlolwa kokusebenza komtholampilo. -

I-SARS-Cov-2 Antigen Rapid Test (Ukusetshenziswa Kobungcweti)
Inkonti encane 500200 Ukucacisa Izivivinyo ezingama-25 / Ibhokisi Isimiso sokutholwa Immunochrochrographic assay Izindola I-Anterior Nasal Swab Ukusetshenziswa okuhlosiwe I-ConfSTep® SARS-Cov-2 Antigen Suptope Cessette isebenzisa ubuchwepheshe be-Immunochromatography ukuthola i-SARS- I-Antigen ye-NUCLEOCACAppID e-Anterior Nasal Swab specimen yabantu. Le testis ukusetshenziswa okukodwa kuphela futhi ihloselwe ukuzihlola. Kunconywa ukusebenzisa lolu vivinyo kungakapheli izinsuku ezi-5 zokuqala uphawu. Isekelwa ukuhlolwa kokusebenza komtholampilo. -
1人份抗原卡实物图唾液版1_00_副本-300x216.png)
I-SARS-Cov-2 Antigen Rapid Test for Saliva
Inkonti encane 500230 Ukucacisa Izivivinyo / ibhokisi / ibhokisi Isimiso sokutholwa Immunochrochrographic assay Izindola AmatheUkusetshenziswa okuhlosiwe Lesi yi-armay esheshayo ye-immunochrographic assay yokutholwa kwe-SARS-Cov-2 Virus NucleocapsopId Protein antiigen eSabiva Swab Swab yomuntu osolwa kusuka kubantu abasolwa abasolwa ngezikhathi ezinhlanu zokuqala kwezimpawu. I-Assay isetshenziswa njengosizo ekutholakaleni kwe-Covid-19. -
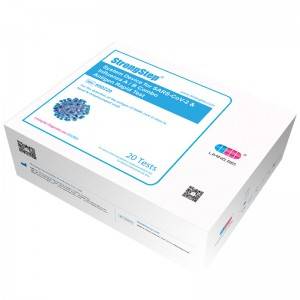
Idivaysi yesistimu ye-SARS-Cov-2 & Umkhuhlane A / B Combo Antigen Rapid Test
Inkonti encane 500220 Ukucacisa Izivivinyo / ibhokisi / ibhokisi Isimiso sokutholwa Immunochrochrographic assay Izindola I-Nasal / Oropharyngeeal Swap Ukusetshenziswa okuhlosiwe Lesi yi-armay esheshayo ye-immunochrographic assay yokutholwa kwe-SARS-Cov-2 Virus NucleocapsopIng Protein Antigen ku-Human Nasal / Oropharyngeal Swab eqoqwe kusuka kubantu abasolwa abasolwa abakhiqizwayo ezinsukwini ezinhlanu zokuqala zokuqala kwezimpawu. I-Assay isetshenziswa njengosizo ekutholakaleni kwe-Covid-19. -

Idivaysi yesistimu ye-Dual Biosafety ye-SARS-Cov-2 Antigen Rapid Test
Inkonti encane 500210 Ukucacisa Izivivinyo / ibhokisi / ibhokisi Isimiso sokutholwa Immunochrochrographic assay Izindola I-Nasal / Oropharyngeeal Swap Ukusetshenziswa okuhlosiwe Lesi yi-armay esheshayo ye-immunochrographic assay yokutholwa kwe-SARS-Cov-2 Virus NucleocapsopIng Protein Antigen ku-Human Nasal / Oropharyngeal Swab eqoqwe kusuka kubantu abasolwa abasolwa abakhiqizwayo ezinsukwini ezinhlanu zokuqala zokuqala kwezimpawu. I-Assay isetshenziswa njengosizo ekutholakaleni kwe-Covid-19. -
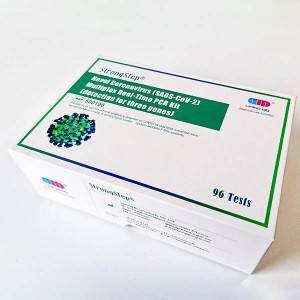
Inoveli coronavirus (SARS-Cov-2) I-Multiplex Real-Time Pcr KIT
Inkonti encane 500190 Ukucacisa Izivivinyo / ibhokisi / ibhokisi Isimiso sokutholwa Bhr Izindola Nasal / nasopharyngeal swab Ukusetshenziswa okuhlosiwe Lokhu kuhloswe ukuthi kusetshenziselwe ukufeza ukutholwa kwe-SARS-cov-2 i-RNA ekhishwe ku-nasopharyngeal swabs, ama-sputum kanye namafutha avela ezigulini ezihlangene ne-FDA / CCR PRTSTS CRTECTION KWI-PDARD CRTECTICTION KAKHULU. I-KIT yenzelwe ukusetshenziswa ngabasebenzi abaqeqeshiwe belebhu
-

AbakwaSars-Cov-2 & Umkhuhlane A / B I-Multiplex Real-Time PCR KCR
Inkonti encane I-510010 Ukucacisa Izivivinyo / ibhokisi / ibhokisi Isimiso sokutholwa Bhr Izindola I-Nasal / Nasopharyngel Swab / OropharyNngeal Swab Ukusetshenziswa okuhlosiwe I-GrostStep ® SARS-Cov-2 & BO / B Multiplex real-time PCR KIT ihloselwe ukutholakala kanye nokwehlukanisa i-SARS-COV-2, umkhuhlane wegciwane kanye nomkhuhlane we-nasal noma ama-OroParyngeal swab stab ama-specimens nezinkambiso eziqoqiwe ze-noral
I-KIT yenzelwe ukusetshenziswa ngabasebenzi abaqeqeshiwe belebhu
-

I-SARS-Cov-2 IGM / IGG Antibody Rapid Test
Inkonti encane I-502090 Ukucacisa Izivivinyo / ibhokisi / ibhokisi Isimiso sokutholwa Immunochrochrographic assay Izindola Igazi eliphelele / serum / plasma Ukusetshenziswa okuhlosiwe Lesi yi-Assay esheshayo ye-immuno-chromatographic assay yokutholwa ngasikhathi sinye kwama-antibodies we-IGM ne-IGG ku-SARS-Cov-2 Virus egazini lonke yabantu, i-serum noma i-plasma. Ukuhlolwa kukhawulelwe e-US ukusatshalaliswa ezindaweni eziqinisekiswe yi-Clia ukwenza ukuhlolwa okunzima.
Lokhu kuhlolwa akukabukezwa yi-FDA.
Imiphumela emibi ayinqamuleli i-acute SARS-Cov-2 Ukutheleleka.
Imiphumela evela ekuhlolweni kwe-antibody akufanele isetshenziselwe ukuthola noma ikhiphe ngaphandle kwe-acute acute SARS-Cov-2 Ukutheleleka.
Imiphumela emihle ingabangelwa ukutheleleka okwedlule noma kwamanje nge-non-sars-cov-2 i-coronavirus strain, njengeCoronavirus Hku1, NL63, OC43, noma i-229E.







